
Spin-off company of the Masaryk University
The Institute of Biostatistics and Analyses of the Masaryk University as an academic entity has been devoted to clinical research project since the early 2000s. With the increasing significance of contract research activities, however, the pressure to provide more professional services was growing. For this reason, Institute of Biostatistics and Analyses Ltd (IBA) was established in 2014 as a spin-off company of the Masaryk University with a share of ownership by the parent institution, taking over activities unrelated to academic grants and education.
Although the history of IBA activities on the market is not very long, the company has an extensive network of supplier and client contacts. These relations were established during a more than 15-year period of contract research activities carried out by the above-mentioned academic entity.
IBA activities are directed at the sector of pharmaceutical industry and life sciences; in this regard, IBA develops and runs information systems for the collection, validation and analysis of clinical data, using both local (desktop) and online (web-based) technologies. These systems, often called electronic data capture (EDC) systems, serve for the collection, management and analysis of data in clinical/health research projects. IBA provides complete services in these projects, including trial or registry design, CRF/eCRF proposal, document completion and communication with regulatory bodies, ensuring expert guarantee by medical societies, data reporting and study monitoring. These services are complemented by a comprehensive support in other areas such as (i) market access, (ii) cost-effectiveness analyses, (iii) pharmacovigilance, (iv) analysis of clinical data including advanced biostatistics, (v) development of tailored software products with a special emphasis on databases and online services, (vi) graphic design.
Institute of Biostatistics and Analyses (IBA s.r.o.) has implemented the integrated system of quality management in accordance with ISO/IEC 20000-1:2006 (guaranteeing the quality of IT services), EN ISO 9001:2016 (quality management systems), and ISO/IEC 27001:2006 (information security management system). Our outputs and services are currently guaranteed by the ISO/IEC 27001 certificate. The quality management system as well as the IT service management system remain implemented, and have been maintained according to the latest versions of the respective standards. The Division of Clinical Projects team also focuses on the management of clinical research projects, and has undergone training in the good clinical practice (GCP).
The point of the above-mentioned standard is to map and to monitor all processes taking place in the organisation, which subsequently facilitates their effective management and control. On top of that, introduction of these standards significantly reduces potential risks occurring from providing IT services and processing data, in terms of technical failure, personnel issues, or even natural disasters.
The three certifications have logical links and mutually complement each other: EN ISO 9001:2016 is a well-known standard of more general characteristics ensuring effective management of the organisation and high quality of products or services. In contrast, ISO/IEC 20000-1:2006 is a relatively new standard focusing on the quality of IT services, their reliability, availability and support for clients in case of problems. Both standards are supplemented with ISO/IEC 27001:2022, which refers to information security management.
In accordance with the requirements of the implemented standards, the IBA issues partial Quality Policies:
IBA's attitude to compliance with ethical rules and its attitude to corrupt practices are expressed in the following documents:
The compliance of our services with ISO 27001 and ISO 9001 has been certified by a globally recognized certification authority – TÜV SÜD.

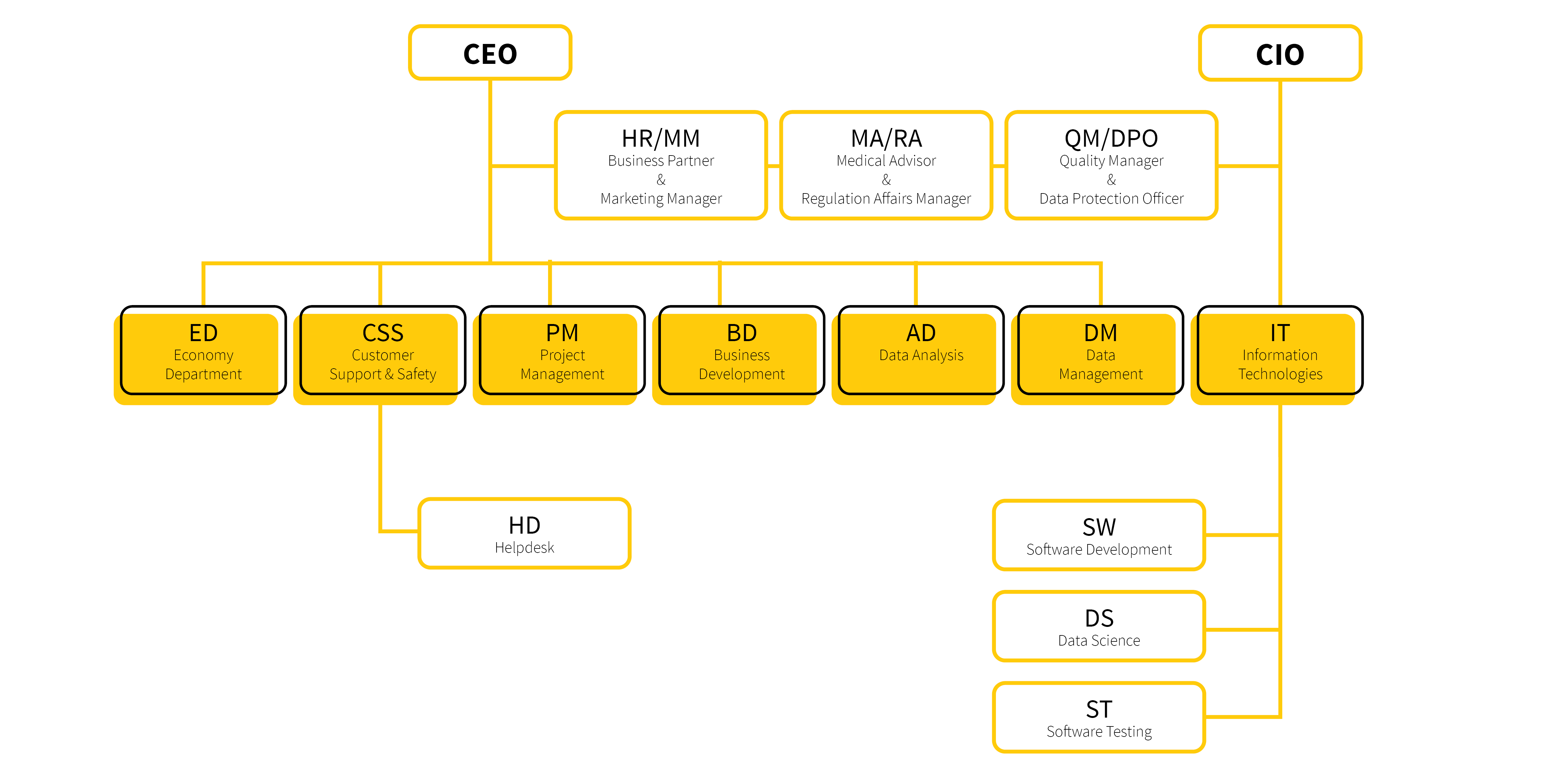
Petr is the chief executive officer (CEO) and one of the company’s founders. He is primarily engaged in searching for new opportunities and in the development of international cooperation. He has been involved in clinical research projects since the early 2000s. During his career, he has participated in hundreds of research projects (both grant-aided and commercial ones) in the areas of oncology, haematology, neurology and rare diseases. He is the author or co-author of numerous research papers. In his own words, articles published in The Lancet Oncology and the Journal of Clinical Oncology were among his biggest scientific achievements.
One of the company’s founders, Daniel is the chief information officer (CIO), mainly overseeing the agile software development. He is an associate professor at the Masaryk University and at the Brno University of Technology, and his research interests are focused on neuroscience, processing and analysis of image data, machine learning and artificial intelligence. As of October 2017, he was the author or co-author of 35 papers in peer-reviewed journals, two monographs and five chapters in books; he participated in the development of 12 engineering works, including one applied certified methodology.


Marika is the Head of Department of Clinical Projects and has been working in the company since 2015. She has been dealing with project management in the pharmaceutical industry and with related issues such as quality management and regulatory affairs since 2003. In her career, she has led dozens of research projects (both grant-aided and commercial ones) focusing on medical devices and medicinal products: from basic research, preclinical and clinical development to placing a product on the market. Her knowledge and experience in time and finance management, as well as keeping documentation in accordance with the quality management system and legislative requirements, are invaluable assets for project management.
Ilona has been working in our company since mid-2015 and as the Head of the Finance Department she is responsible for financial management. She is involved in accountancy and tax issues and in overseeing company’s economy status. She has gained experience as an accountant, financial manager, and particularly as an economic expert in a multinational company.


David is the head of Data Management department. He graduated from the Brno University of Technology in the field of Bioinformatics and Biocomputing. He joined our company in 2016 as a clinical data manager as a regular team member. Currently he leads the data management team, where they create research databases and registries, revise CRF proposals, create eCRF in our CLADE-IS system, migrate and transform data, control data quality, participate in on-line visual analytics, etc. David emphasizes work efficiency, its automation and especially the friendly atmosphere within the team.
Michal is the head of the Data Analysis department. He has been working for the company since the summer of 2017. He has been analyzing clinical data since 2014, and is the author or co-author of a number of scientific publications, mainly in the field of pneumology and cardiology. During his work in clinical and experimental research, he participated in many projects, which included study design, selection of appropriate statistical methods, analysis of data, writing reports and publications. In academia, he participates in teaching biostatistics at Masaryk University.


David is the leader of software developers. He joined our company during his university studies and gradually worked his way up to the software development department. At first, he focused mainly on data transformations, reporting and analytical components of our key product CLADE-IS, and later seamlessly penetrated all its key components and modules. Currently, in addition to programming, he also mentors a young team of developers and testers, with an emphasis on adhering to the principles of object-oriented programming and clean code.
The Supervisory Board is the supervisory authority of IBA Ltd. The Supervisory Board is obliged to supervise the company’s management, participates in strategic decisions relevant to the company and takes initiatives for the company’s future operation.
Eva Janouškovcová, PhD
Chairman of the Supervisory Board, Head of Technology Transfer Office, Masaryk University
Assoc. Prof. Regina Demlová, PhD
Member of the Supervisory Board, Head of Department of Pharmacology, Masaryk University
Marta Valešová, MSc, MBA
Member of the Supervisory Board, Bursar of Masaryk University
Prof. Valentine Provazník, MSc, PhD
Member of the Supervisory Board, Head of the Department of Biomedical Engineering, Faculty of Electrical Engineering and Communication Technologies, Brno University of Technology; Member of the Scientific Council, Brno University of Technology
Prof. Petr Štourač, MD, PhD
Member of the Supervisory Board, Head of Department of Paediatric Anaesthesiology and Resuscitation, Masaryk University and University Hospital Brno; Vice-Dean for Development and Studies in Clinical Fields, Faculty of Medicine, Masaryk University
The current ownership structure consists of three associates, and the Masaryk University is one of them. Therefore, the Masaryk University has still the possibility to influence the company’s management and operation, it has the right to call a meeting of associates and to propose measures.
The Institute of Biostatistics and Analyses is an investigator of the project “Extending services portfolio of an information system for the data management in clinical research”. The project is supported by the OP EIC from the European Union funds.
Main objective of the project is the development of a platform for composition of information systems focused on data management and data mining in the field of pharmaceutical industry and life sciences. Offer of digital services based on a developed and validated information system will make reliable analytical models accessible to the companies for their decision-making and precise information sources for regulatory bodies. The project steps out of a laboratory environment of clinical trials and focuses on the evidence supported by real-world data.