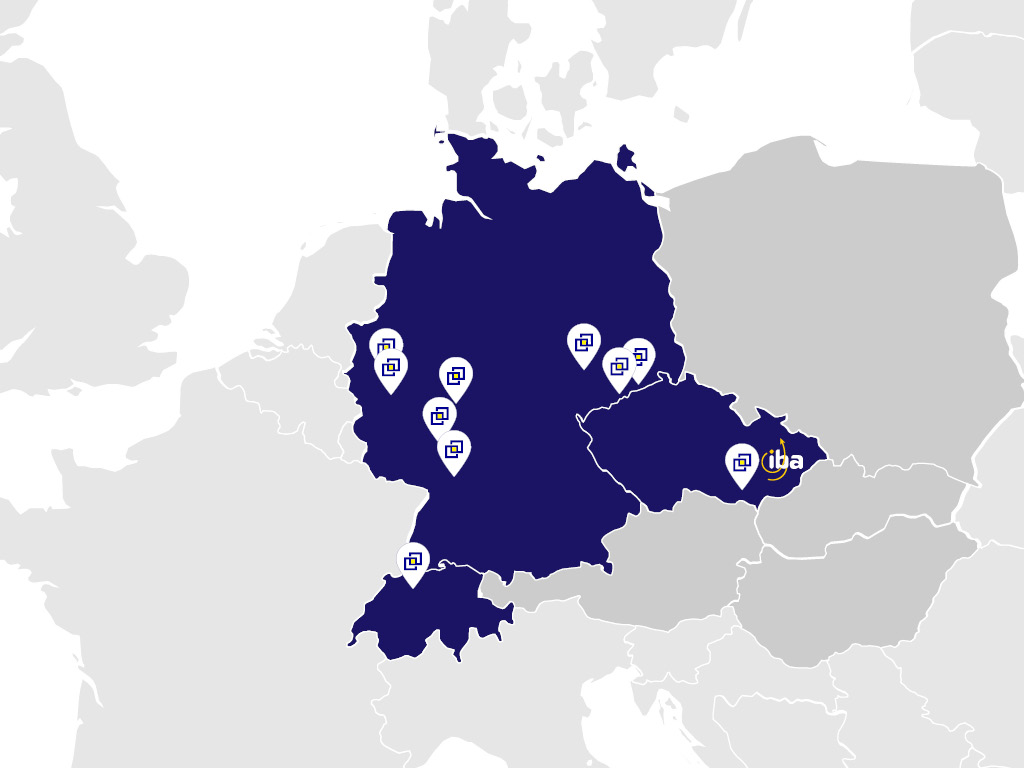
On September 10th, we achieved a significant milestone by receiving certification from the European Clinical Research Infrastructure Network (ECRIN)
On September 10th, we achieved a significant milestone by receiving certification from the European Clinical Research Infrastructure Network (ECRIN), a goal we had been diligently preparing for over the past year. We are proud to announce that we are now the 22nd certified data center within the ECRIN network. In Central Europe, only institutions in Germany and Switzerland have earned this prestigious certification.
Based on recommendations from the audit, we further enhanced several internal processes, particularly in the validation of our CLADE–IS system. This step not only helped us meet the strict certification requirements, but also significantly improved the efficiency and quality of our services.
We are excited about the new opportunities and possibilities that collaboration within the ECRIN network will bring. We believe this certification will open doors to new projects and partnerships, benefiting not only our organization but the entire scientific community. Collaboration with other certified centers will allow us to share knowledge and experiences, driving further development and innovation in clinical research.
17. 9. 2024
The Institute of Biostatistics and Analyses is an investigator of the project “Extending services portfolio of an information system for the data management in clinical research”. The project is supported by the OP EIC from the European Union funds.
Main objective of the project is the development of a platform for composition of information systems focused on data management and data mining in the field of pharmaceutical industry and life sciences. Offer of digital services based on a developed and validated information system will make reliable analytical models accessible to the companies for their decision-making and precise information sources for regulatory bodies. The project steps out of a laboratory environment of clinical trials and focuses on the evidence supported by real-world data.