Clinical research services
Clinical research services
Planning a clinical research project and need expert support or guidance? We are here to help. With our extensive experience, qualified team, advanced technological infrastructure, and proprietary electronic data capture software, we can efficiently ensure the execution of your project. In addition to study implementation, we offer consulting services to help tailor your project to regulatory requirements and streamline processes such as market entry. Our offering includes comprehensive professional services, expert consulting, technological support, and management of ethical and legal aspects of clinical projects.
“Experience and advice from those who have faced similar challenges are the value you seek. Together, we are smarter. Stronger.”
Pharmaceuticals
We provide comprehensive services for non-interventional studies (NIS) and patient registries. Our team offers expert consultations on pharmaceutical regulations, including Directive 2001/83/EC, the EU Clinical Trials Regulation (2014/536), and Regulation 2004/726.
Non-interventional observational studies (NIS)
We carry out non-interventional drug studies for both commercial partners and academic institutions. Our aim is to provide a real-world view of the effectiveness, safety, and impact of approved medicines in routine practice, aligned with the study’s research question.
Patient registries
We efficiently manage and implement patient registries using our in-house data capture system, CLADE-IS, which meets the highest standards of quality and data security. These registries collect and store data on individuals under medical supervision for a common reason, helping us gain deeper insights into the health conditions and trends within specific patient populations.
Clinical trials
We conduct clinical trials using our proprietary electronic data capture system, CLADE-IS. The goal of these trials is to collect high-quality scientific data and evaluate the efficacy, safety, and side effects of treatments, drugs, or therapeutic interventions on patient health.
Medical devices
Pre-market clinical studies
We offer comprehensive support for pre-market clinical studies of medical devices in compliance with the EU Medical Device Regulation (EU MDR 2017/745). We help you generate robust evidence on the safety and performance of your medical device before market launch. Our approach ensures that all critical aspects — such as functionality, accuracy, reliability, and biocompatibility — are thoroughly evaluated. We ensure the assessment meets regulatory requirements, covering both technical parameters like measurement precision and device longevity, as well as user experience factors including usability and patient comfort.
Post-market clinical follow-up (PMCF)
We provide end-to-end support for post-market clinical follow-up to monitor the ongoing safety and effectiveness of medical devices, in accordance with the EU MDR (2017/745). Our goal is to ensure the long-term performance of your devices through continuous data collection, monitoring, and analysis.
In vitro diagnostics
Pre-market clinical studies for in vitro diagnostic devices
We offer comprehensive support for pre-market clinical studies of in vitro diagnostic (IVD) devices in accordance with the EU In Vitro Diagnostic Regulation (IVDR 2017/746). These studies are designed to ensure that diagnostic devices meet the required safety, performance, and quality standards for their intended purpose before being placed on the market, and to verify regulatory compliance. Our goal is to help you meet all legal and regulatory requirements for entering the EU market.
Post-market performance follow-up (PMPF)
We provide full support for the post-market monitoring of in vitro diagnostic devices, in compliance with EU IVDR (2017/746). This ongoing monitoring is essential to maintain regulatory compliance and ensure the safety of patients and device users. We help you keep your IVD devices aligned with EU IVDR requirements while ensuring their long-term safety and performance.
Our services include
Regulatory advisory and consulting
Need help navigating the complex regulatory environment of clinical trials and medical devices? We’ll guide you to achieve your goals in full compliance with current regulations.
“Follow the standards, master the quality.”
Project management
Looking to efficiently plan and manage your project? We’ll create a timeline, assign tasks, secure resources, and ensure smooth communication so everyone involved has the right information at the right time.
“Turning hypotheses into results.”
Feasibility studies
Unsure whether your project is feasible? We’ll help you with a thorough analysis—assessing technical, organizational, methodological, financial, and legal aspects, identifying risks, and evaluating available resources and potential benefits.
“Explore the possibilities, unlock the opportunities.”
Clinical data analysis
Have clinical data that needs analyzing? We’ll process it for you, answer your research questions, identify trends, and provide a solid foundation for informed decisions within your study or research.
“Turn data into insights and knowledge.”
Clinical data management
We take care of clinical data collection, validation, and management to ensure it is accurate, secure, and well-organized.
“Organized data – reliable results.”
Pharmacovigilance
Our pharmacovigilance team provides comprehensive monitoring and analysis of adverse drug reactions to ensure patient safety and regulatory compliance.
“Monitor and protect.”
Medical writing
We create clear, scientifically sound, and accessible content—from posters and reports to collaborative publications. Need help with a scientific text? We’re here for you.
“Think clearly, write clearly.”
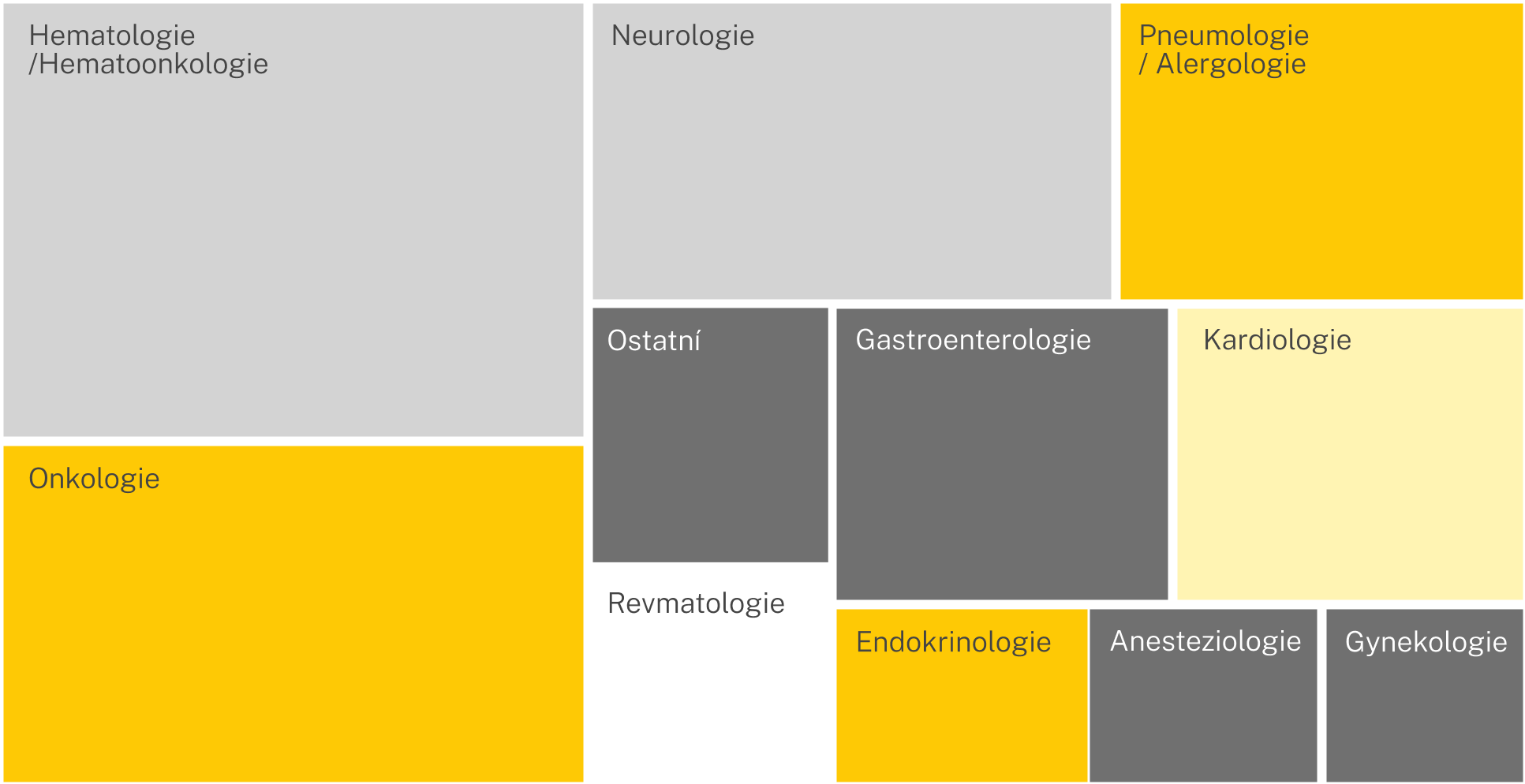
Clinical disciplines
We have experience across a wide range of clinical disciplines, and our portfolio continues to grow.
Clinical research service guides
Clinical data services for MDR compliance
Want to be sure your medical device meets all the requirements of the Medical Device Regulation (MDR)? Don’t waste time searching for the right procedures — we’ve already done that and know the system inside out. We’ll help you reach your goals and stay compliant with the latest regulations. Whether you’re preparing to enter the market or conducting a routine review, we’ll help you stay MDR-compliant — without stress or unnecessary complications. Learn more in our guide Clinical Data Services for MDR Compliance: Ensuring Compliance and Improving Quality
The role of real-world evidence (RWE) in clinical research
If you work in healthcare, pharma, medical device development, clinical data management, or clinical project management, this guide could reshape how you see clinical research. Unlocking the Power of Real-World Data in Clinical Research: A Compelling Value Proposition explores the tools, techniques, and experts you need to run successful real-world evidence (RWE) projects — and shows why real-world data is an essential asset in your toolkit.







